Endoscopy
On average, 19.98 % of reprocessed gastrointestinal endoscopes may be contaminated when used in patients and varies between different geographies (PubMed Central june 2022)

Biofilms & endoscopy
” The utility of lighted magnification and borescopes for visual inspection of flexible endoscopes”
Visible damage and residue or debris were observed in 100% of 25 endoscopes at both assessments … read more hereGrowth of biofilms inside endoscope channels can result in failure of endoscope reprocessing and is an important factor in the pathogenesis of endoscopy-related infections.6
Microbial surveillance to monitor endoscopes after reprocessing has been recommended by a majority of organizations (ASGE, ESGE, ESGENA, GESA).
Countries with systematic endoscope monitoring confirm high contamination rates.
6. Buss et al., 2008, Kovalera et al., 2009
Why worry about endoscopes ?
10 to 30% of patient-ready endoscopes are contaminated with microorganisms.1
If contaminated scopes are used, investigators noted high rates of transmission.²
More outbreaks are linked to contaminated endoscopes than to any other medical device.³
Endoscopes not sterilized, hence reprocessing has narrow margin of safety. Any slight deviation can lead to survival of microorganisms.⁴
Endoscopes are significant vectors for nosocomial transmission of microorganisms as a result of scope contamination from the patient or from the inanimate environment (e.g. automated endoscope reprocessing devices).⁵
1. Direction Générale de la Santé, France, mar 2007
2. Kovaleva et Al., 2013
3. CDC Guidelines for disinfection and sterilization in healtcare facilities, 2008
4. Alfa et al. 2006. American Journal of Infection Control, 34 (9), 561-570
5. Alfa quoting Spach et al.
Infection & endoscopy
- Infection rates “far higher” than expected in US endoscopy centers.
- An update on gastrointestinal endoscopy-associated infections and their contributing factors.
- IF IT’S NOT CLEAN, IT SHOULDN’T BE DISINFECTED
- Inorganic and organic materials interfere with the effectiveness/ antimicrobial activity of disinfectants and sterilization.²
- It is impossible to disinfect an inadequately cleaned instrument.²
- “Any disinfection process is doomed to fail if cleaning is inadequate.”⁷
2. Kovaleva et Al., 2013
7. CSS, BE: Recommandations d’entretien du matériel endoscopique et de prévention des infections –2010
Other applications

endoscopy
10 to 30% of patient-ready endoscopes are contaminated with microorganisms.

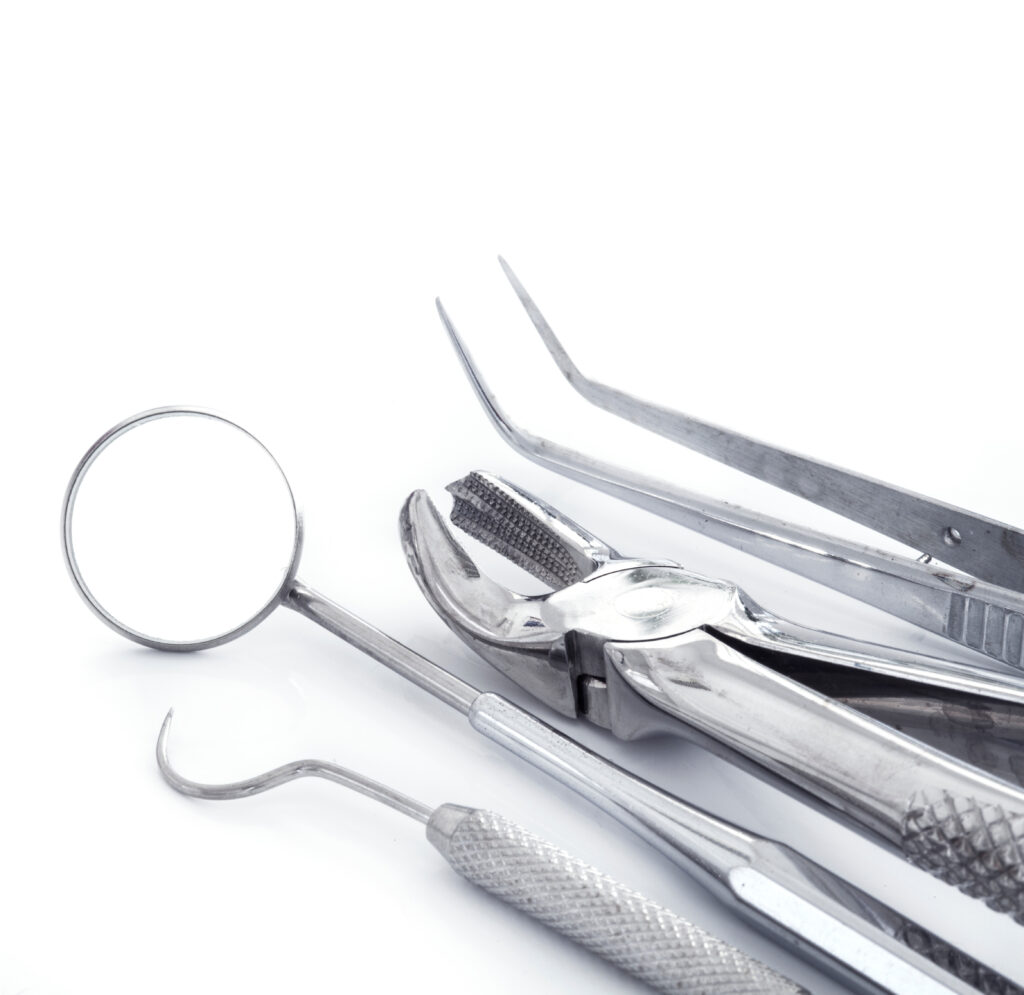
surgical instruments
Over 60% of correctly-processed surgical instruments exhibit residual protein soiling.

surfaces
Working in a safer & cleaner environment is a must. Take control.