Why are biofilms an issue ?
Up to 99% of bacteria exist in the form of biofilm.
Mature biofilms continuously contaminate their environment by randomly releasing microorganisms.
65%
Biofilms are responsible for 65% of all infections.
resistance
is favored inside biofilms due to horizontal transfer of genes carrying antibiotics-resistance.
medical devices
are subject to contamination by biofilms.
cleaning is crucial
It is impossible to disinfect or even sterilize inadequately-cleaned medical devices.
tolerance x1000
Bacteria are up to 1000x more tolerant to biocides if in a biofilm.
The solution ?
Enzymatic decontamination
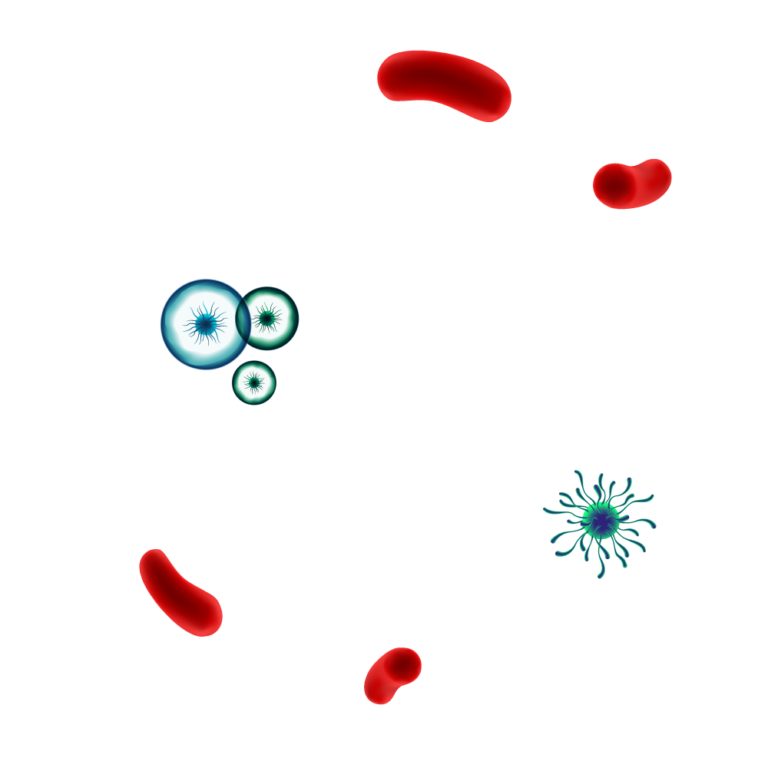
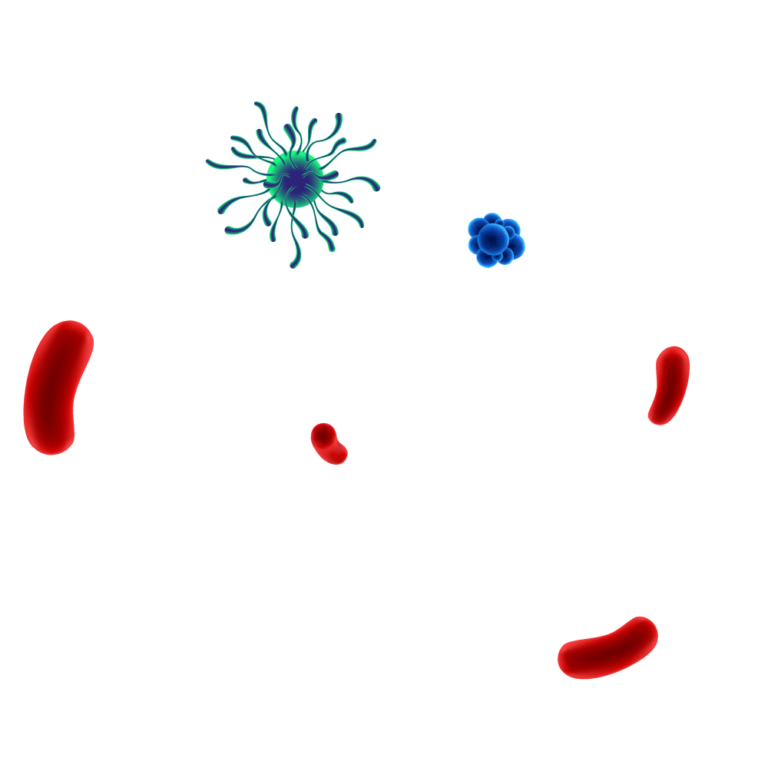
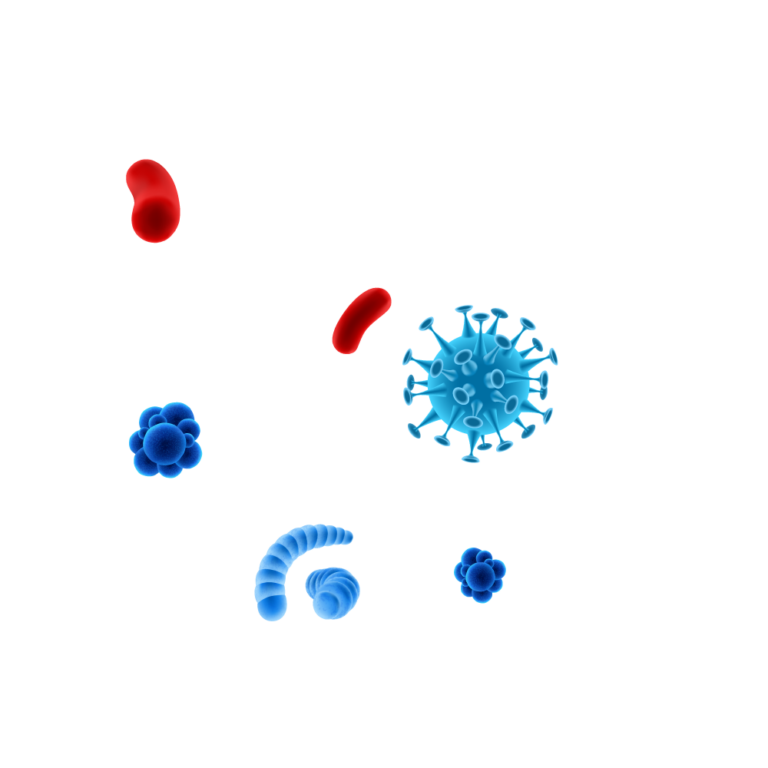
Pathogens are protected by
the biofilm matrix.


Destructive action on
the biofilm matrix.
Enzymatic action dissolves the biofilm matrix exposing pathogens & allowing for an effective disinfection
Why is enzymatic cleaning the best choice ?
Enzymes, safer & more efficient


more efficient
In depth and longer lasting cleaning assured.
safer
For staff, patients and visitors.

cost saving
Direct and indirect costs.

more sustainable
Materials, surfaces and environment.

more efficient
In depth and longer lasting cleaning assured.

safer
For staff, patients and visitors.

cost saving
Direct and indirect costs.

more sustainable
Materials, surfaces and environment.
Enzymes vs Traditional chemistry
Unlike traditional chemistry that lifts and holds soil particles in suspension, good enzyme detergents also dissolve soil in an irreversible reaction.
Enzymes are not degraded by their activity and are more effective for complex medical devices where mechanical action, like brushing, is difficult or some parts are inaccessible.
Applications

endoscopy
10 to 30% of patient-ready endoscopes are contaminated with microorganisms.

surgical instruments
Over 60% of correctly-processed surgical instruments exhibit residual protein soiling.

surfaces
Working in a safer & cleaner environment is a must. Take control.
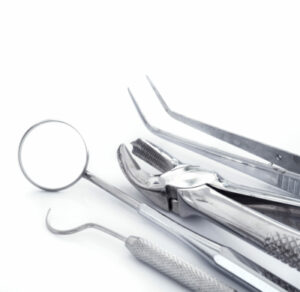
dental applications
Dental instruments are possible agents for pathogen transmission.

Take control
Our enzymatic detergents are patented for dissolving Biofilms (EP2789682A1), infection treatment (BE 2020/5726, WO2017211737) and surface cleaning (EP2243821).

Enzymatic activity (Protease) expressed as mg/ml Trypsin equivalent of 9 commercially available concentred Medical Device cleaners.
OneLife offers proven effectiveness
We design, develop & produce enzymatic solutions that contribute to the prevention of Antimicrobial Resistance (AMR) and Healthcare Acquired Infections (HAI).
Our solutions are safe and effective, the best in class to break down biofilms and have a long lasting deep cleaning effect.
OneLife’s enzymatic technology enables complete and irreversible degradation of organic soil and biofilms.
Their performance is tested on biofilms’ broad spectrum of bacterial strains.

OneLife detergents ensure medical devices decontamination and optimal infection risk management.
Preventive routine treatment, integrated into the existing decontamination protocols, continuously removes all soil, preventing re-formation of biofilms.